
Infusions every 8 weeks after 3 induction doses. REMICADE® is administered by intravenous (IV) infusion over a period of not less than 2 hours.1
5 mg/kg IV given at 0, 2, and 6 weeks
as an induction regimen
5 mg/kg IV given every 8 weeks thereafter
as a maintenance regimen
Before infusing REMICADE®, provide patients or their caregivers with the Medication Guide for REMICADE®. You can obtain a printable PDF of the full Prescribing Information and the Medication Guide here. Refer to the Preparation and Administration Instructions and important information on what to do in the event your patient experiences an infusion reaction.
REMICADE® is intended for use under the guidance and supervision of a healthcare provider. The supplied lyophilized powder must be reconstituted and diluted prior to administration. The infusion solution should be prepared and administered by a trained medical professional using aseptic technique by the following procedure:

Calculate the dose, total volume of reconstituted REMICADE® solution required, and the number of REMICADE® vials needed. More than one vial may be needed for a full dose.
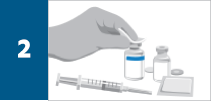
Reconstitute each 100-mg REMICADE® vial with 10 mL of Sterile Water for Injection, USP, to obtain a concentration of 10 mg/mL, using a syringe equipped with a 21-gauge or smaller needle as follows:
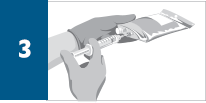
Dilute the total volume of the reconstituted REMICADE® solution to 250 mL* with sterile 0.9% Sodium Chloride Injection, USP, (do not dilute with any other diluent) as follows:
*For volumes greater than 250 mL, either use a larger infusion bag (eg, 500 mL) or multiple 250-mL infusion bags to ensure that the concentration of the infusion solution does not exceed 4 mg/mL.
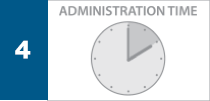
The REMICADE® infusion should begin within 3 hours of reconstitution and dilution. The infusion must be administered intravenously for at least 2 hours with an infusion set with an in-line, sterile, non-pyrogenic, low-protein-binding filter (pore size of 1.2 µm or less).

Given that the vials do not contain antibacterial preservatives, discard any unused portion of the infusion solution (do not store for reuse).
No physical biochemical compatibility studies have been conducted to evaluate the co-administration of REMICADE® with other agents. REMICADE® should not be infused concomitantly in the same intravenous line with other agents.
Infusion reactions may occur with numerous IV medications, including REMICADE®. In clinical trials of REMICADE®, the majority of infusion reactions were mild to moderate. Most of these reactions were manageable and responded to appropriate treatment steps.
Serious cerebrovascular accidents, myocardial ischemia/infarction (some fatal), hypotension, hypertension, and arrhythmias have been reported during and within 24 hours of initiation of REMICADE® infusion. Cases of transient visual loss have been reported during or within 2 hours of infusion of REMICADE®. Monitor patients during infusion and if serious reaction occurs, discontinue infusion. Further management of reactions should be dictated by signs and symptoms.
Thorough patient assessment and screening for hypersensitivity reactions are key to helping to prevent infusion-related events.
Prior to treatment, ensure appropriate personnel and medication are available to treat reactions (eg, hypersensitivity, other reactions) that occur during infusion and shortly after infusion. Prior to infusion with REMICADE®, patients may be premedicated with histamine-1 receptor antagonists, histamine-2 receptor antagonists, acetaminophen, and/or corticosteroids.
For mild to moderate reactions during the infusion, consider slowing or stopping the infusion. Upon resolution of these reactions, may reinitiate at a lower infusion rate and/or with histamine-1 receptor antagonists, histamine-2 receptor antagonists, acetaminophen, and/or corticosteroids. Discontinue the infusion if the mild to moderate reactions reoccur.
Discontinue the infusion if severe hypersensitivity reactions occur during the infusion.
Reference: 1. REMICADE® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.